Background: Acute Chest Syndrome (ACS) is the greatest contributor to the morbidity and mortality of Sickle Cell Disease (SCD) patients with a rate of 12.8 cases per 100 patient-years. The mortality rates of ACS are 4 times higher in adults than in children (Vichinsky et al, Blood, 1997). The root cause of ACS in SCD is related to endothelial dysfunction (Paul et al, European Journal of Hematology 2011). While existing therapies target infection, inflammation, and alveolar hypoxia, they do not address endothelial dysfunction, a major contributor to SCD associated ACS. Defibrotide is a polydisperse mixture of predominantly single stranded oligonucleotides derived from porcine intestinal mucosa. Several pre-clinical studies indicate that defibrotide primarily protects endothelium, particularly in small vessels, and reduces endothelial cell injury (Falanga et al, Leukemia, 2003) (Cairo/Cooke et al, British Journal of Haematology 2020). In a randomized phase III study, a decrease in incidence of SOS/VOD was observed in pediatric hematopoietic stem cell transplantation patients who received prophylactic defibrotide (Corbacioglu et al, Lancet, 2012). We hypothesized that Defibrotide would be safe and well tolerated in children and adolescents with SCD-associated ACS.
Primary Aims: To determine the safety and efficacy of defibrotide in the treatment of patients with SCD-associated ACS.
Design/Methods: Patients with SCD- (Homozygous Hemoglobin S disease, Hemoglobin SC Disease or Hemoglobin Sβ 0/+ Thalassemia) associated ACS 2 to 40 years of age were enrolled. Defibrotide, generously supplied by Jazz Pharmaceuticals, was administered at 6.25 mg/kg IV Q6H (total daily dose 25 mg/kg/day) within 24 hours of consent and continued for 7 days or until the patient was discharged from hospital (IND 127812) (NCT 03805581). All patients were treated with current standard of care, including antibiotics, analgesics, oxygen, intravenous fluids, and packed red blood cells (PRBC)/exchange transfusion. Complete blood counts and coagulation studies were performed at baseline, day 7 and day 30, while chest radiograph (CXR), CT chest angiogram (CTA), pulmonary function test, and transthoracic echocardiogram were performed at baseline and 30 days after defibrotide was first given. Blood plasma was collected from patients at baseline, day 7, day 30, and age matched SCD patients without ACS and analyzed for biomarkers of inflammation through ELISA and Luminex multiplex assays and significance was calculated using two-tailed T tests.
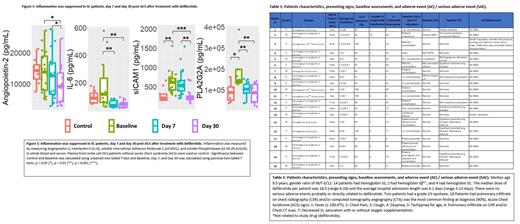
Results: We have enrolled 20 patients (median age 9.5 years) with a gender ratio of M/F 8/12. Patients had hemoglobin SS (n=14), hemoglobin Sβ 0/+ (n=2), or hemoglobin SC (n=4). The median doses of defibrotide per patient was 18.5 (range 6-28) and the average hospital admission length was 6.1 days (range 3-13 days). There were no serious adverse events possibly, probably, or directly related to defibrotide, as judged by investigators. Two patients had a grade I/II epistaxis that did not disrupt defibrotide's course. Pulmonary infiltrate on CXR and/or CTA was the most common finding at diagnosis, detected in 18/20 study participants. Thirteen patients had fever as a presenting symptom, with an average fever duration of 2.8 days (range 1-6 days). Nine patients required O 2 supplementation, with an average duration of 4.1 days (range 2-7 days). Thirteen patients were treated with blood transfusions with a median of 1.9 units (PRBC unit was defined as 15ml/kg) transfused per patient (range, 1-5 units) and 1 patient underwent an exchange transfusion (Table 1). Soluble Intercellular Adhesion Molecule-1 (sICAM-1) was significantly elevated at the onset of ACS (p < 0.01) compared to control SCD plasma and was reduced by Day 30 after defibrotide treatment. Other markers of inflammation, including Angiopoetin-2 (Ang2), Interleukin-6 (IL-6), and Phospholipase A2-IIA (PLA2G2A) were elevated at baseline in patient plasma samples but were significantly decreased following defibrotide treatment (p < 0.05, 0.01, 0.01, respectively) (Figure 1).
Conclusion: Our data suggests that defibrotide is safe and well tolerated in SCD patients with ACS. sICAM-1, Ang2, IL-6, and PLA2G2A may serve as important biomarkers in SCD associated ACS. A randomized phase II/III multi-center clinical trial will need to be conducted to further investigate efficacy of defibrotide in patients with SCD-associated ACS.
OffLabel Disclosure:
Cooke:Jazz Pharmaceuticals: Consultancy. Cairo:Omeros Pharmaceuticals: Consultancy, Research Funding; Celularity: Research Funding; Astra Zeneca: Honoraria; Merck: Research Funding; Miltenyi Biotec: Research Funding; Sobi: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Amgen Inc.: Honoraria, Speakers Bureau; Servier Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy.
Defibrotide is a polydisperase mixture of single stranded oligonucleotides. Several pre-clinical studies indicate that defibrotide primarily protects the endothelium and appears to reduce endothelial cell injury. Sickle cell disease associated acute chest syndrome is rooted in endothelial dysfunction, making defibrotide a potential therapeutic.